Localizable fluorescent ion indicator platform for high throughput organellar ion channel assays
Not every drug target is expressed on the plasma membrane. As a matter of fact, 90% of cellular membranes reside inside the cell, and 70% of membrane proteins can be found on these organellar membranes. The ability to probe ion channels and transporters on their native organellar membranes opens up immense possibilities for novel drug discovery.
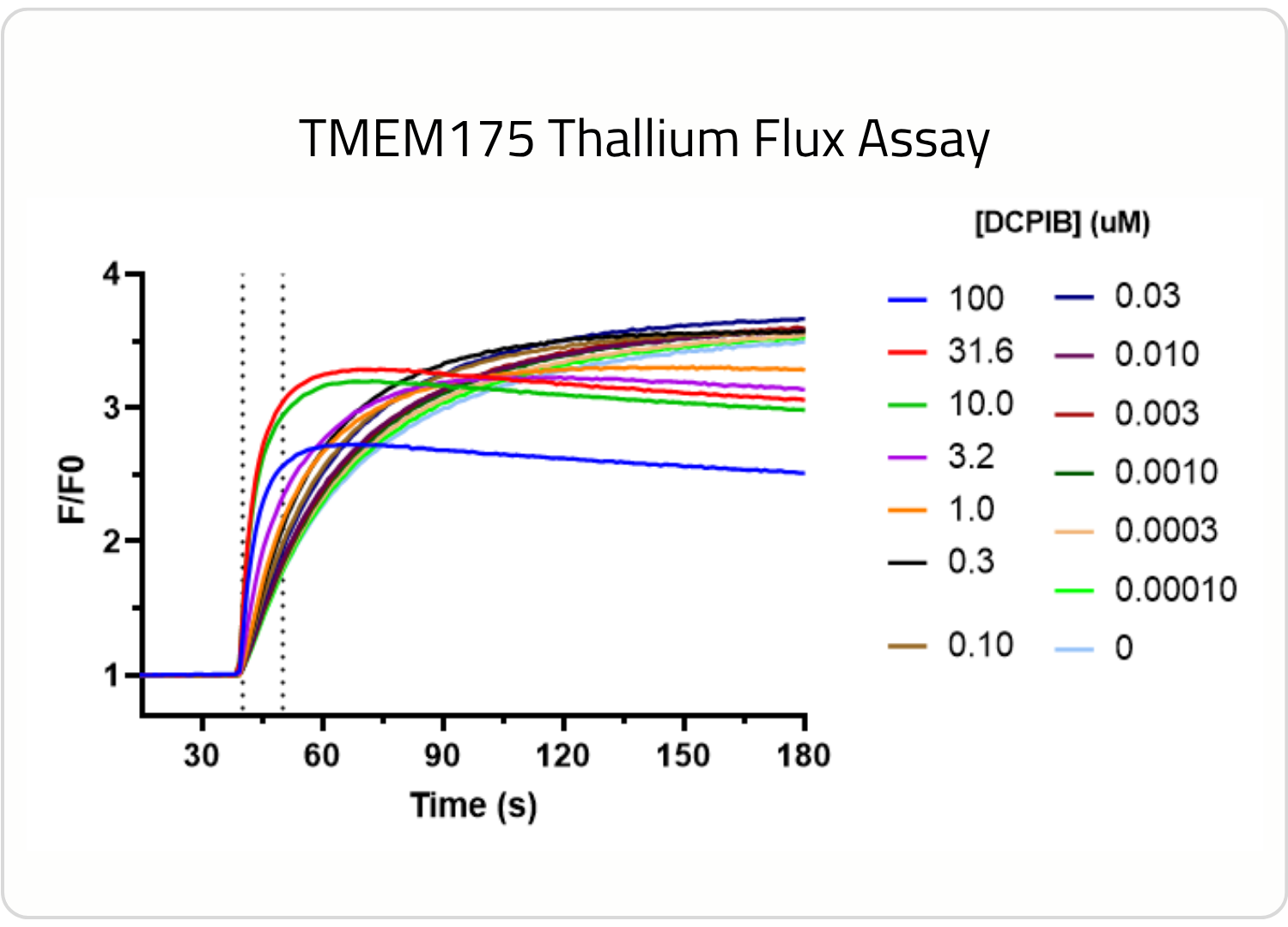
Investigating the pharmacology of your target of interests on its native membrane is of paramount importance. Protein conformational changes can be caused by lipid membrane composition, membrane thickness, membrane curvature, and the environment surrounding either side of a membrane, leading to significant changes in the function and pharmacology of membrane bound proteins. TMEM175, a lysosomal cation channel associated with Parkinson’s Disease, conducts potassium ions and preferentially conducts protons at lysosomal pH (4.5-5.5). We’ve developed a TMEM175 cell line and TMEM175 assay using thallium flux to further explore its pharmacology, and this is just one example that highlights why it’s important to measure the activity of your target on its native membrane.
Moreover, compounds must engage the pharmacological target on its resident membrane to be effective. A compound that makes it into the transmembrane domain of the plasma membrane, may not be able to engage the same target on an intracellular membrane.This loss of potency can be caused by the compounds inability to traffic to intracellular sites, or be driven by conformational changes in SAR. The ability to measure ion channel and transporter activity on organellar membranes inside of fully healthy cells in HTS campaigns provides more relevant and meaningful insights for hit identification and lead optimization studies.
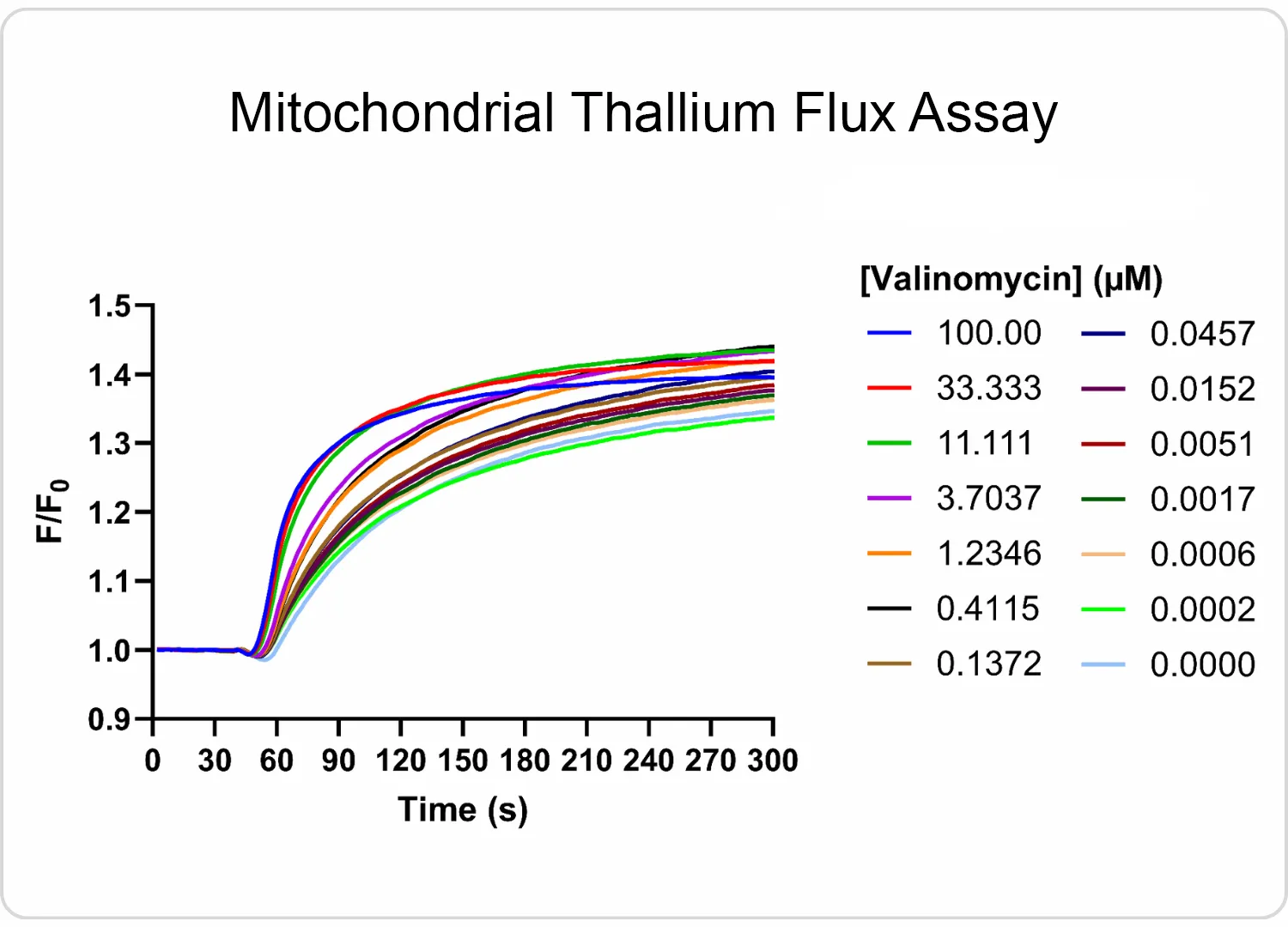
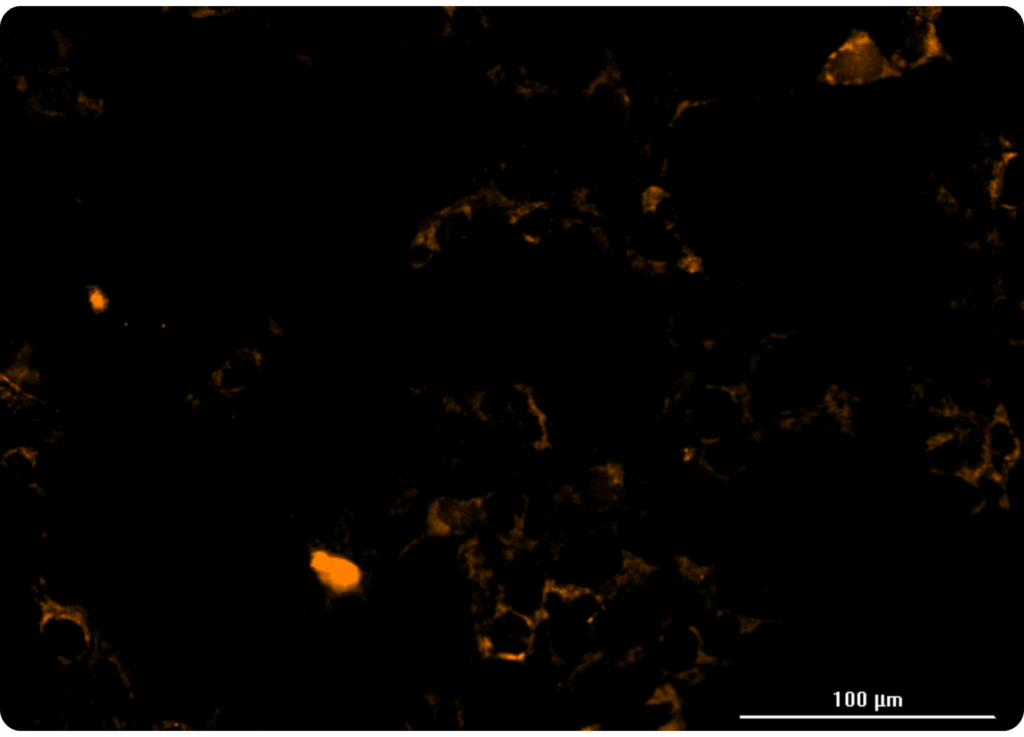
Our HaloTag™platform provides a high-throughput screening compatible solution for measuring organellar ion channel and transporter activity. Our HaloTag™-compatible collection of fluorescent ion indicators enables us to localize fluorescent probes to individual organelles, and only monitor ion flux across the desired organellar membrane. We’ve demonstrated this capability inside a handful of organelles to date, including nuclei and mitochondria. At right, you can see one example of a mitochondrial thallium flux assay in HeLa cells expressing HaloTag™ in mitochondria treated with various concentrations of valinomycin. Kinetic data was captured in a 384-well format on a FLIPR instrument.
A localizable thallium indicator that can be selectively targeted to individual cells or organelles using HaloTag® technology. ION Thallos-HTL is a ligand for the HaloTag® enzyme. It can be used to conduct cell-specific potassium channel assays in mixed cell populations, or to probe organellar ion channels in their native environment. Cells expressing HaloTag® are required when using ION Thallos-HTL.
A localizable thallium indicator that can be selectively targeted to individual cells or organelles using HaloTag® technology. ION Thallos Gold-HTL is a ligand for the HaloTag® enzyme, and displays red-shifted excitation and emission relative to ION Thallos-HTL. It can be used to conduct cell-specific potassium channel assays in mixed cell populations, or to probe organellar ion channels in their native environment. Cells expressing HaloTag® are required when using this product.
BAPTA JF™549-HTL is a calcium indicator that also displays the Halotag ligand for cell-specific and organellar localization using Halotag technology. A Janelia Fluor® dye backbone provides excellent brightness and photostability, making it useful for measuring fast calcium dynamics in neurons and cardiomyocytes. This calcium indicator also outperforms red-shifted genetically encoded calcium sensors in sensitivity and fluorescence intensity.
More on the way…
1) Cells are engineered to express HaloTag™ in an organelle of interest
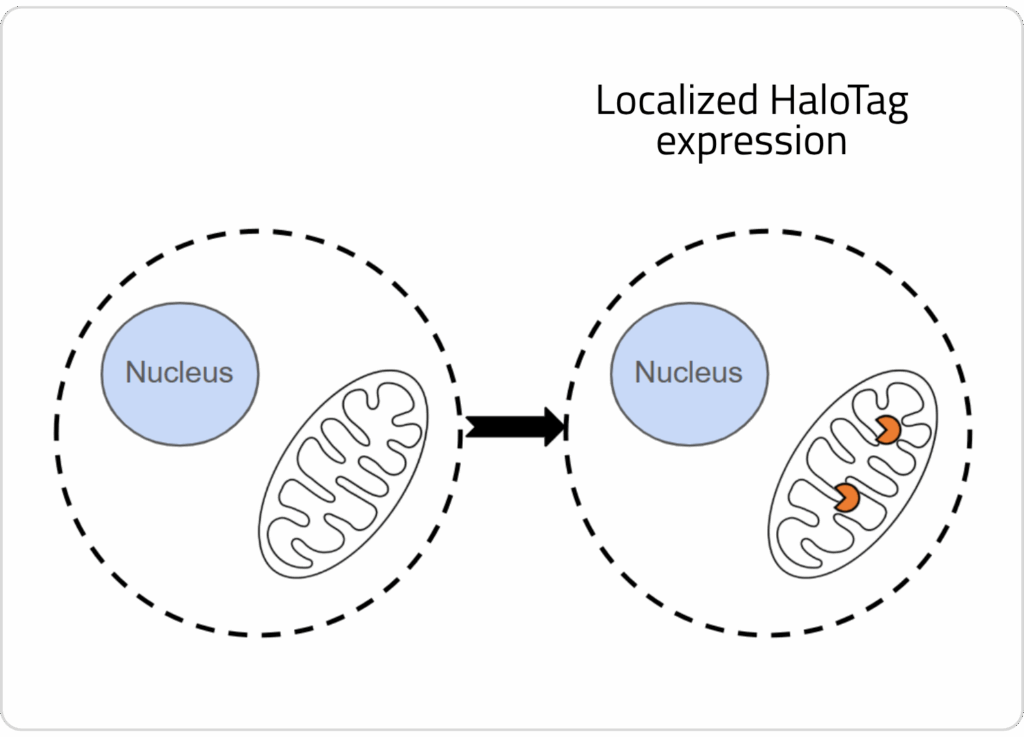
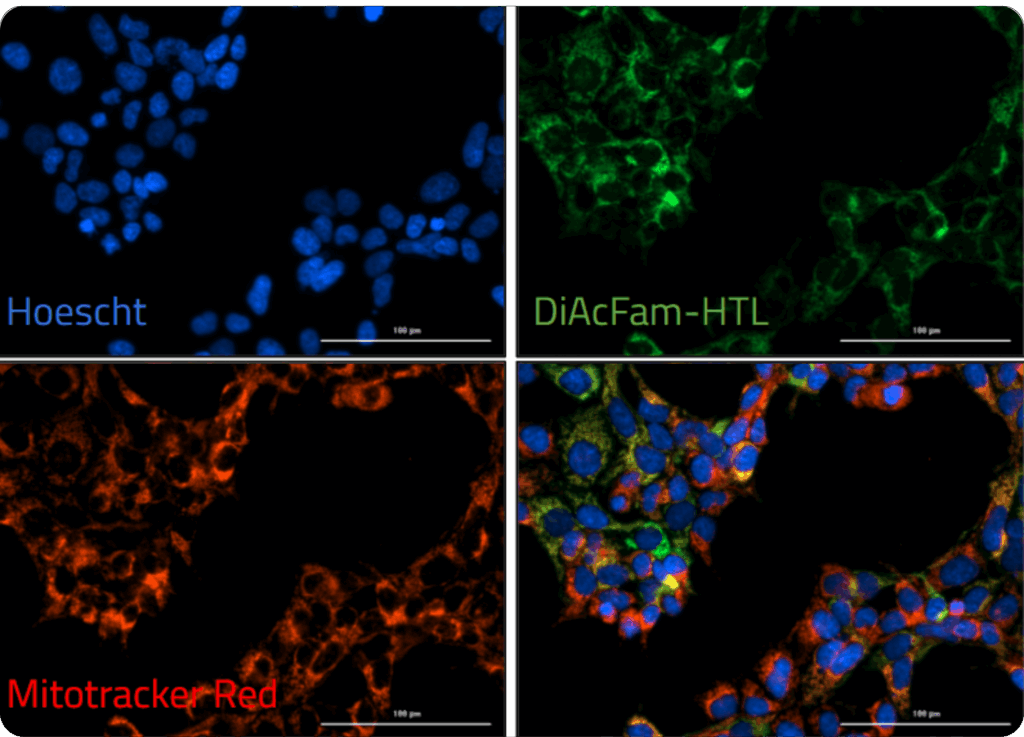
2) One of our HaloTag™-compatible ion indicators is loaded into cells, where the sensor covalently binds to the enzyme in the organelle of interest.
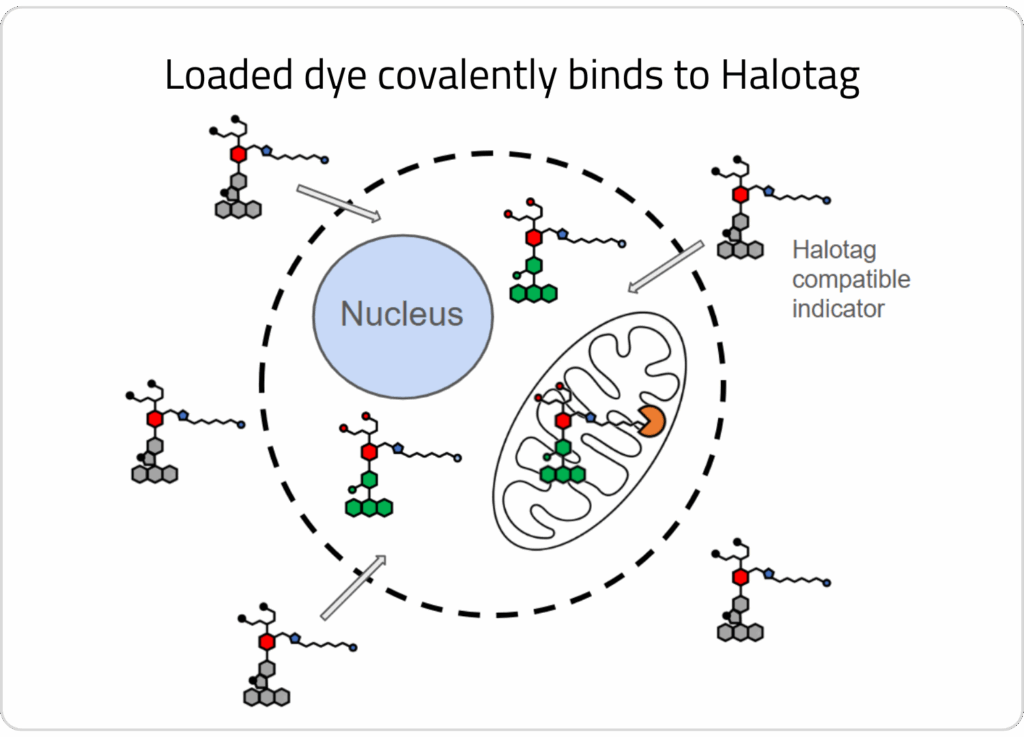
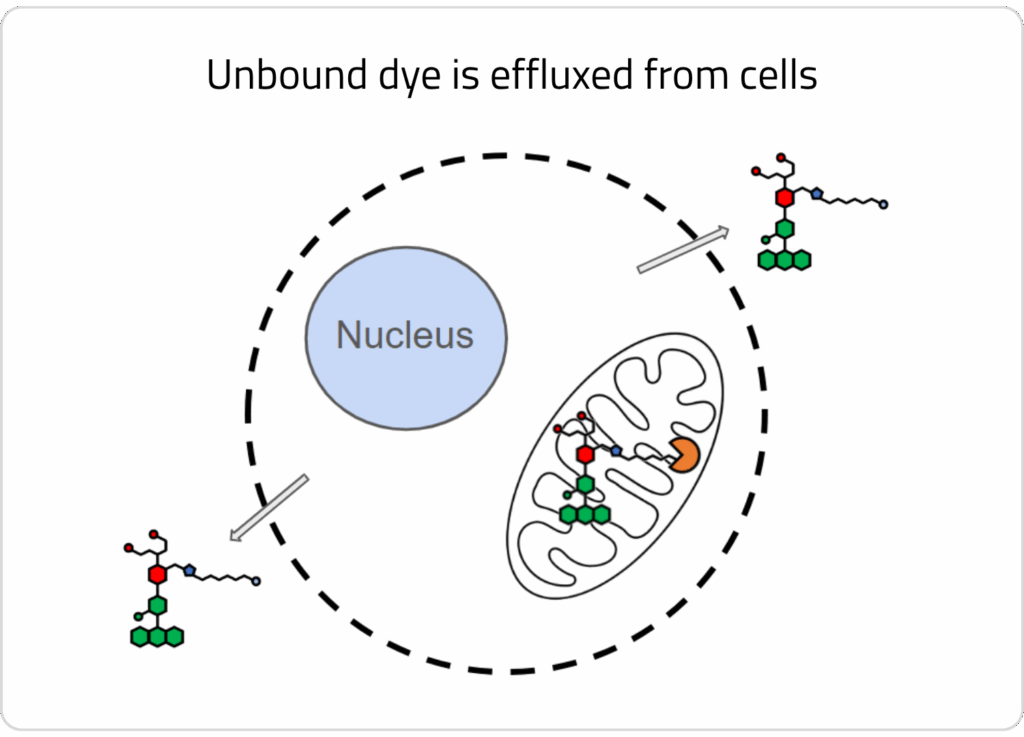
3) A wash step is conducted to allow unbound dye to efflux out of cells. HaloTag™-bound dye remains localized in the target organelle.
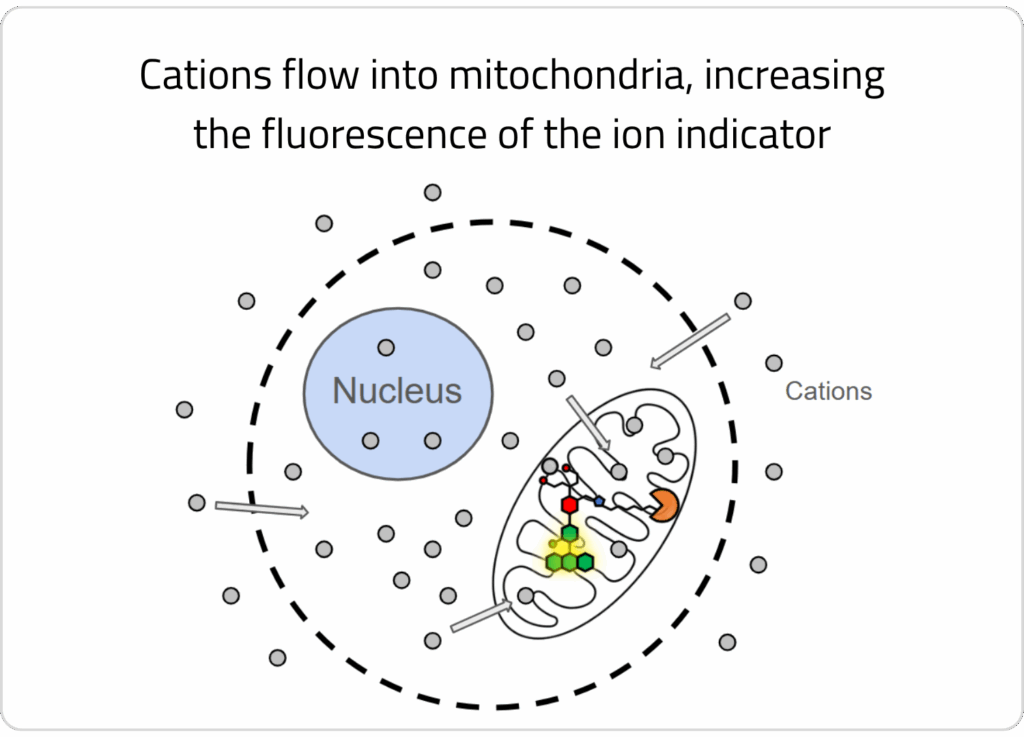
4) A stimulus solution is added that elicits a change in ion concentration across the organellar membrane, which is detectable by the localized ion indicator.
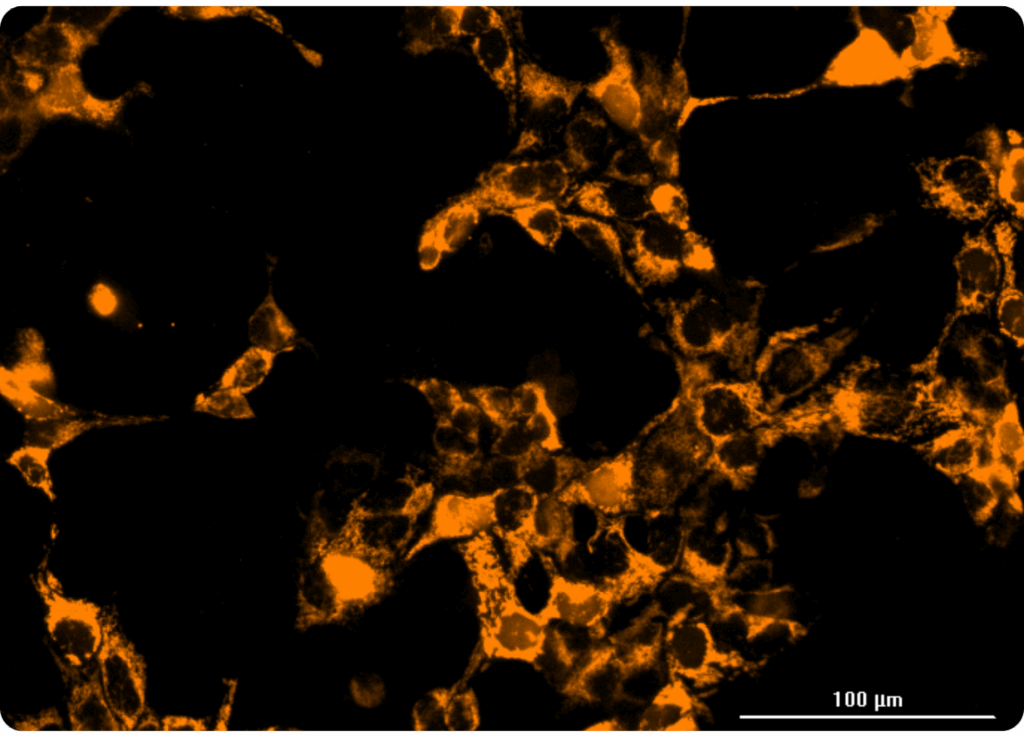
This is an entirely new realm for organellar ion channel drug discovery, enabling screening of intracellular ion channels on their native membranes inside of a cell. Better yet, the technology is compatible with high throughput screening platforms, unlocking immense potential to discover modulators of these important therapeutic targets.